Tatmyctofusp: More Than a Molecule, A Platform

- Essential core: At the heart of the platform is MYC, a master regulator of cell growth, metabolism, and effector function.
-
Adaptable across products: Deployed in diverse formats
including:
- Krysaygio™ – an ex vivo T cell therapy for lung conditions.
- Tatmyctotrans-E™ – an ex vivo cell therapy enhancer.
- Tatmyctotrans-A™ – in vivo direct injection to patients, as adjuvant to other therapies.
- Treactivus™ – a direct injectable for veterinary use.
- Broad applicability: Enhances T cells, NK cells, and potentially other immune cell types, supporting CAR-T, TCR-T, NK cell therapies, and general ex vivo conditioning.
- Dual modality: Functions both as a standalone therapeutic (directly restoring immune function) and as an ancillary enhancer (optimizing ex vivo or in vivo cell therapies).
- Self-limited action: Naturally degrades within ~5 days, offering strong but reversible effects without the risks of permanent genetic edits.
- Extending to stem cell applications: Preclinical work shows Tat-MYC can also support hematopoietic stem cell survival, engraftment, and immune reconstitution across cord blood, G-CSF–mobilized peripheral blood, and bone marrow sources, opening a new frontier beyond immuno-oncology.
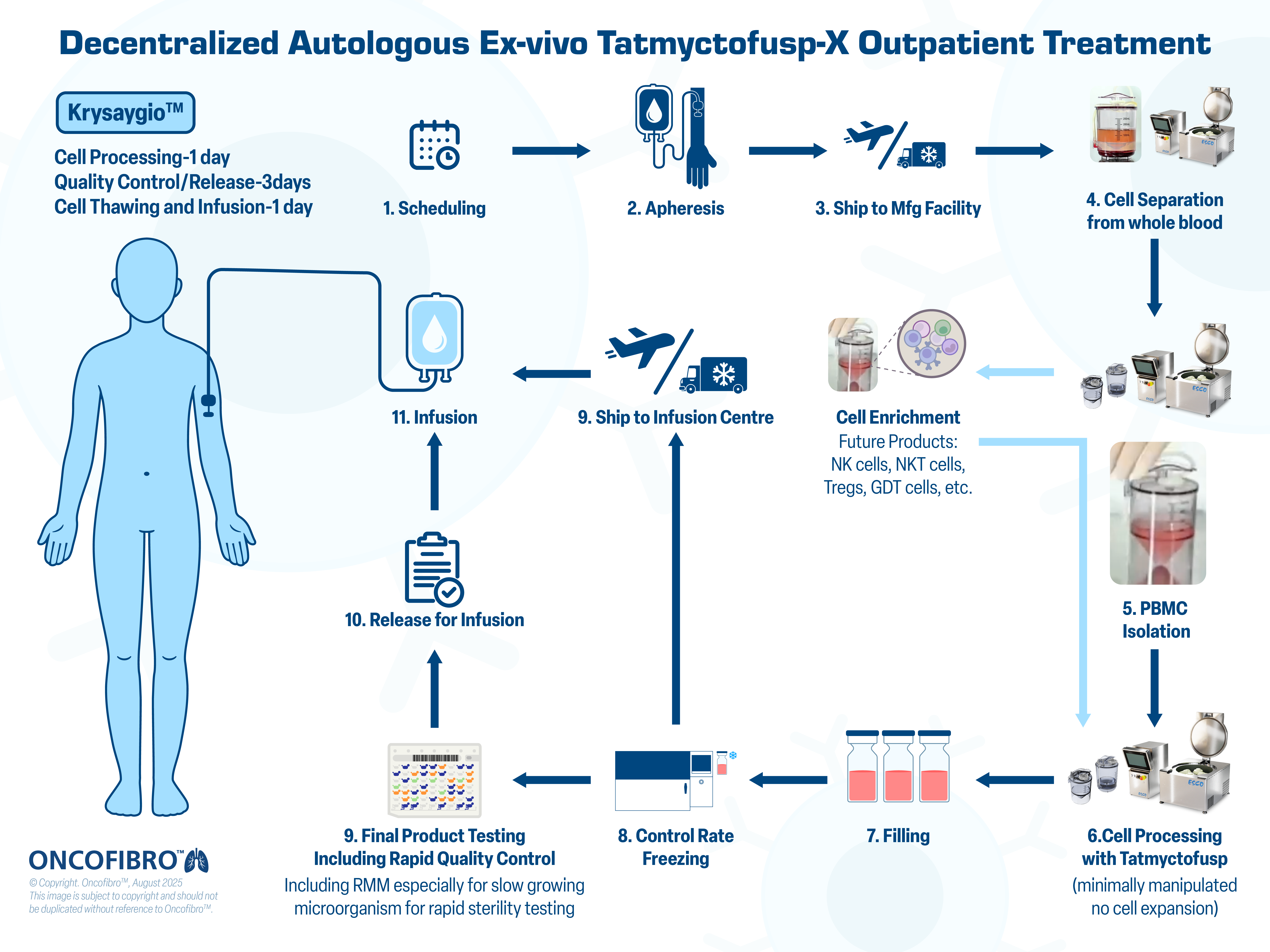
Decentralized Autologous Ex-vivo Tatmyctofusp-X Outpatient Treatment
At Oncofibro, we are pioneering the future of cell therapy with a scalable, decentralized, and fast approach – delivering reactivated T cells within a median vein-to-vein time, with rapid quality control and release of just five days.
- Cell Processing – 1 day
- Quality Control / Release – 3 days
- Cell Thawing and Infusion – 1 day